

An orbital can hold 0, 1, or 2 electrons only, and if there are two electrons in the orbital, they must have opposite (paired) spins. This systematic organization is related to the number of electrons in a neutral atom, called the atomic number, Z. Aufbau Principle - electrons fill orbitals starting at the lowest available energy state before filling higher states (1s before 2s). The periodic table of the elements groups elements with similar properties into columns. The physical and chemical properties of elements are directly related to the number of electrons a neutral atom has. In 1925, the Austrian physicist Wolfgang Pauli (see Figure 1) proposed the following rule: No two electrons can have the same set of quantum numbers. Pauli exclusion principle: no two electrons in the same atom can have the same set of four quantum numbers. *Note: there is currently no known element whose electrons occupy the g subshell.All atoms except hydrogen are multiple-electron atoms. Total Number of Electrons in the n th shell (2n 2) or 2(total number of orbitals in the shell) In simple terms, the Pauli exculsion principle paired electrons to have opposite spins. This means an orbital can hold a maximum of two electrons, and then the electrons must have opposite spins, +1/2 and -1/2. Let's summarize the number of electrons held in Shells and Subshells of n= 1 to n=5. No two electrons in a atom can have an identical set of four quantum numbers. The principle was originally proposed by Austrian physicist Wolfgang Pauli in 1925. An orbital may only hold a maximum of two electrons at a time, so they must have diametrically opposed spins. The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f subshell has 7 orbitals with 14 electrons. The Pauli Exclusion Principle asserts that no two electrons in an atom or molecule can have the same electronic quantum numbers. So, this tells us that each subshell has double the electrons per orbital. The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. In both cases, n=2, l=0, m l=0, but the orange electron has an m s= +1/2 and the blue electron has an m s=-1/2. It provided a mechanism to explain the variety and behavior of the. To correct this, we represent one electron as pointing up and the other is pointing down. The Pauli exclusion principle enabled the quantum structure of the atom to be understood. In the first example, the 2 electrons would have the same m s quantum number, and therefore, will be incorrect, since no two electrons are exactly alike. One electron is spin up (m s = +1/2) and the other would spin down (m s = -1/2).

A single orbital can hold a maximum of two electrons, which must have opposing spins otherwise they would have the same four quantum numbers, which is forbidden. As an example of the Pauli Exclusion Principle in action, we might consider a neutral helium atom.
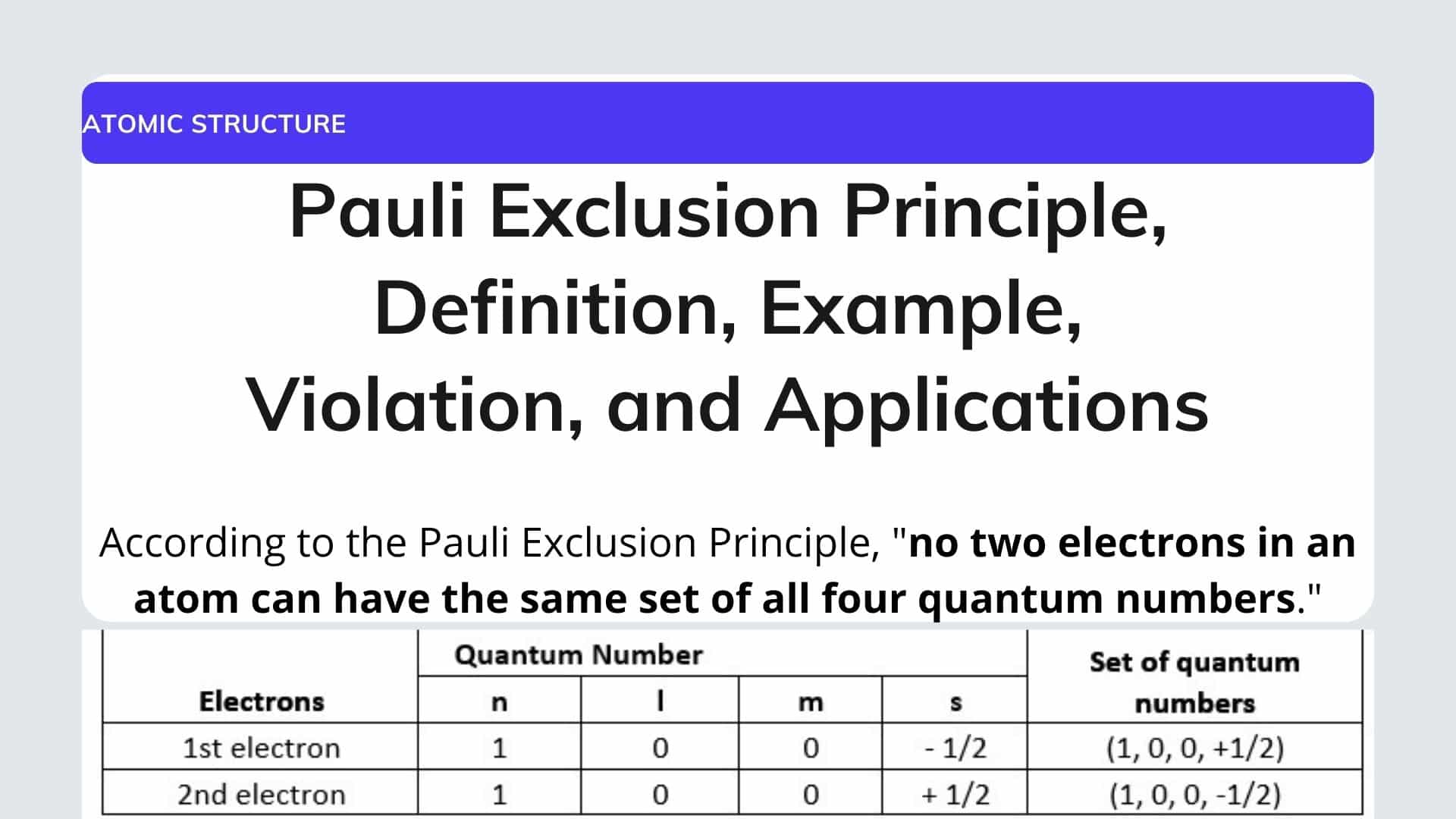
Example of the Pauli Exclusion Principle. The first three (n, l, and m l) may be the same, but the fourth quantum number must be different. For most applications in chemistry, the rule is used to explain or identify the electron shell structure of atoms and to anticipate which atoms are most likely to contribute electrons.

The Pauli exclusion principle states that no two electrons can have the same four quantum numbers.


 0 kommentar(er)
0 kommentar(er)
